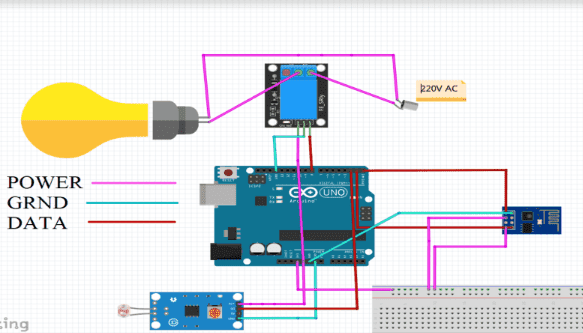
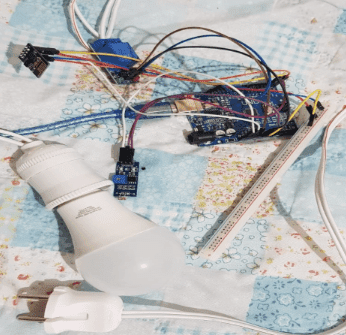
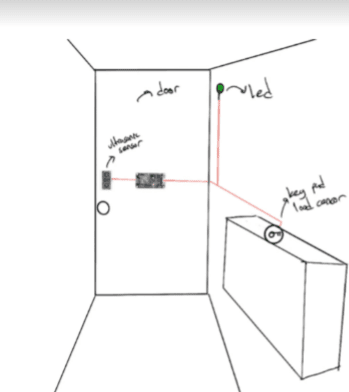
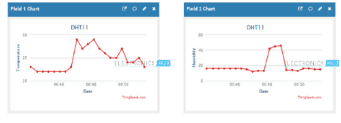
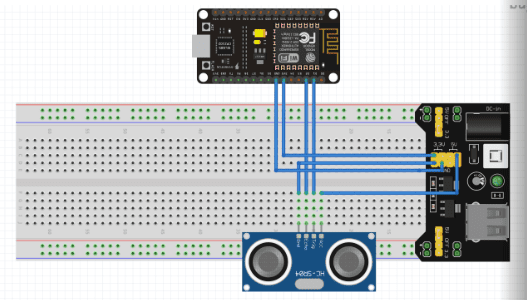
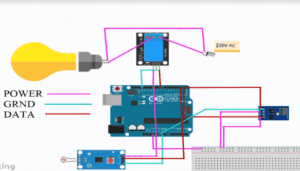
Description
Today’s people have begun to use technology in every aspect of their life so
quickly adapted to technology. With the ease and reliability brought by technology, people
started to shape their life. Recently, the interaction of objects has made technology more
attractive. Through the Internet, objects can send data and commands to each other. In this
way, the work or measurements are reflected to people through a screen. One of the reasons
for focusing on technology so fast is that people can secure their lives in some areas. Many
people die due to the explosion or poisoning of the natural gas every year. Natural gas is used
in homes, workplaces or in many areas. It is used by filling natural gas tanks or transporting
by pipes. High concentrations of LPG vapor may cause unconsciousness or death, even for a
short time in enclosed or non-ventilated environments because when high concentrations of
gas are inhaled, heart rhythm disorders may occur. Carbon monoxide is found in the fumes of
carbonaceous fuels such as unburned natural gas. Carbon monoxide is a molecule of carbon
and an oxygen atom. It is a colorless, odorless, tasteless and non-irritating gas, so its presence
is not recognized. Carbon monoxide is released as a result of incomplete combustion of
carbon bearing compounds. It's a very strong poison. If the density in the inhaled air
increases, it passes into the blood and connects to the hemoglobin where oxygen is
transported more easily than oxygen and it is not easily separated.. Carbon monoxide (CO)
poisoning remains one of the most common causes of death due to poisoning in developed /
developing countries around the world. Every year, especially in winter, poisoning from CO
is quite high. An adult person loses his life as soon as possible if he / she remains closed in a
room containing 1% CO for half an hour. Another danger is the risk of explosion by
accumulation of natural gas in the environment. Natural gas is a gas that is lighter than air. In
case of a gas leak, it becomes harmless by mixing into the atmosphere from the places left for
ventilation. Risk of explosion if natural gas accumulates only in a closed environment. It can
explode when it comes into contact with fire or sparks if mixed with air in the closed volume
of 5-15%. The objective of this work to prevent the loss of life and property caused by gas
leakage.